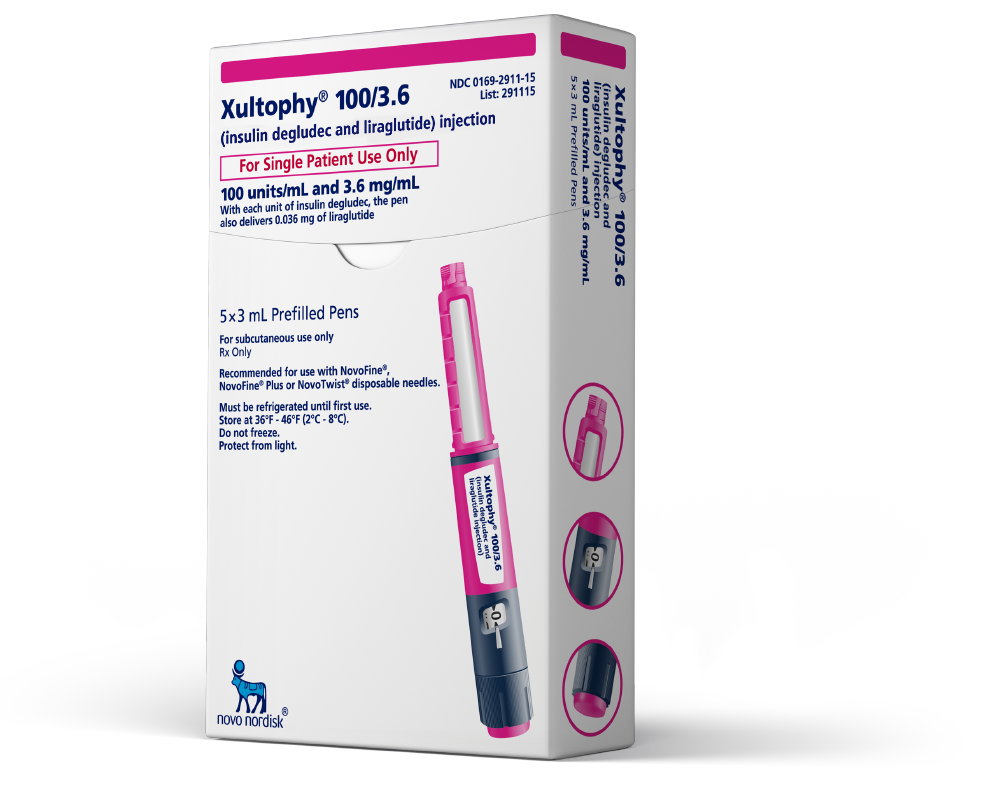
Pharmacist Information | Xultophy® 100/3.6 (insulin degludec and liraglutide) injection 100 u/mL and 3.6 mg/mL

Novo Nordisk issues voluntary nationwide recall of Levemir®, Tresiba®, Fiasp®, Novolog® and Xultophy® product samples due to improper storage temperature conditions | Markets Insider

Novo Nordisk issues voluntary nationwide recall of Levemir®, Tresiba®, Fiasp®, Novolog® and Xultophy® product samples due to improper storage temperature conditions