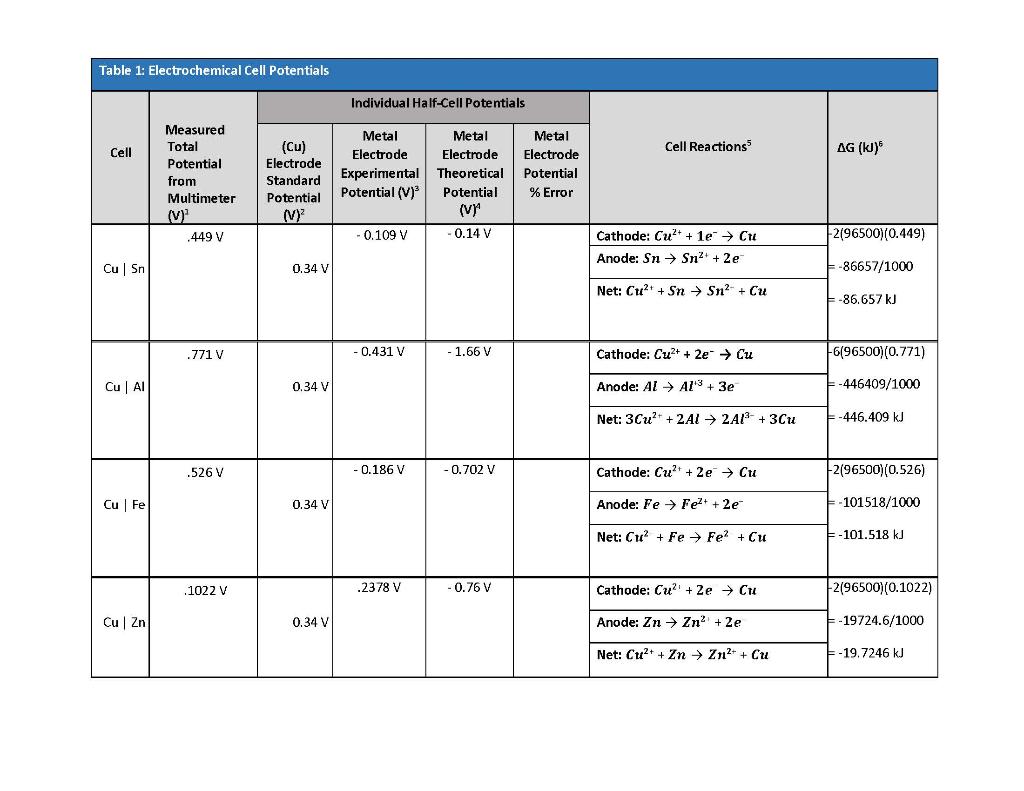
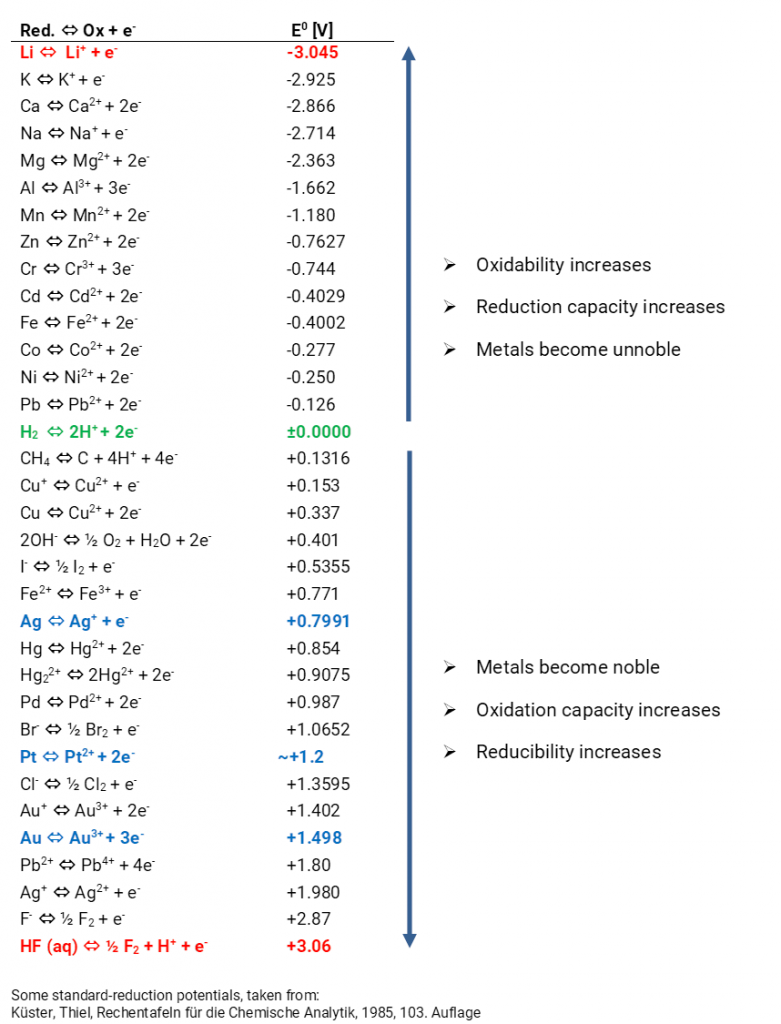
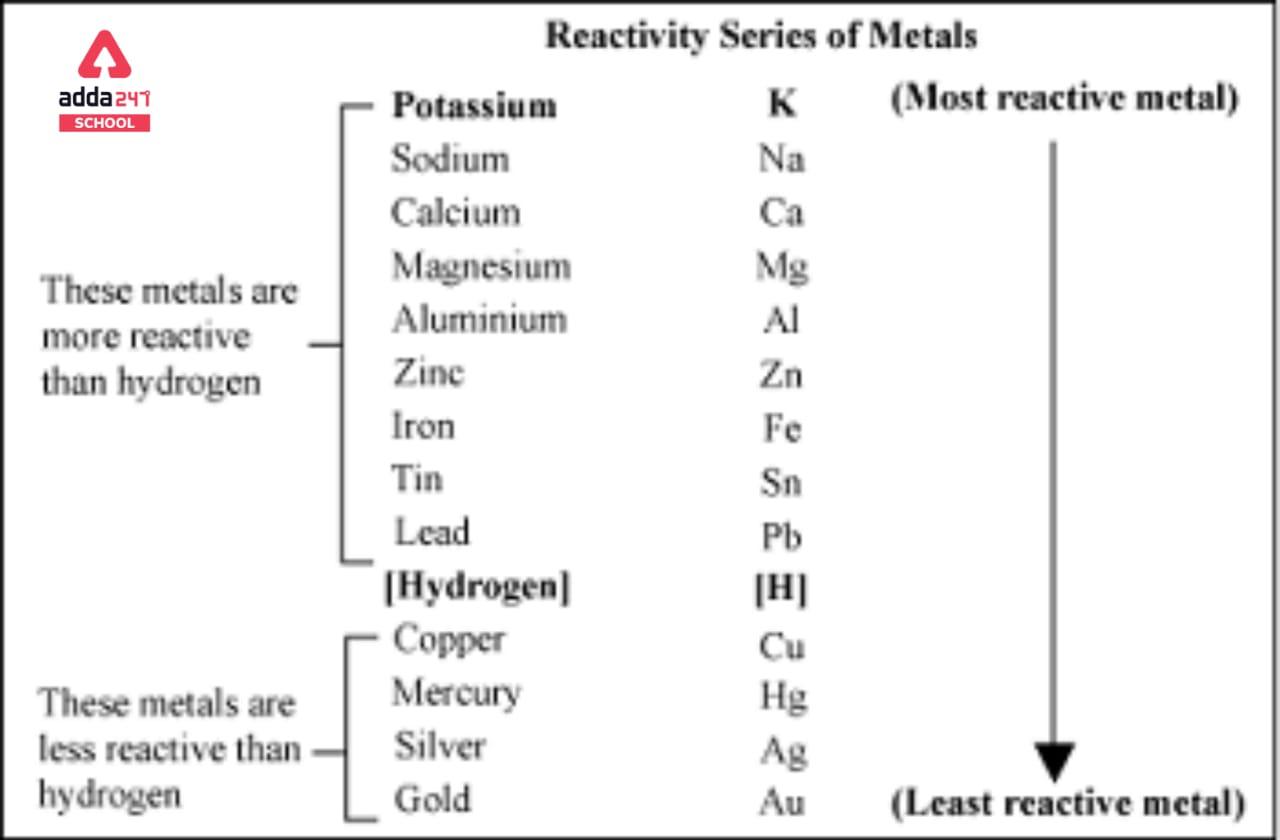
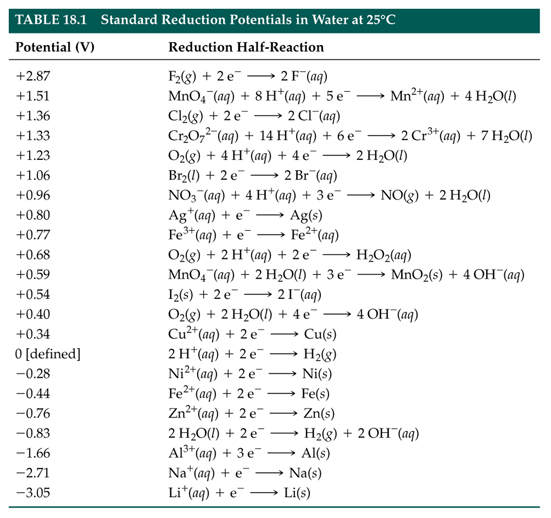
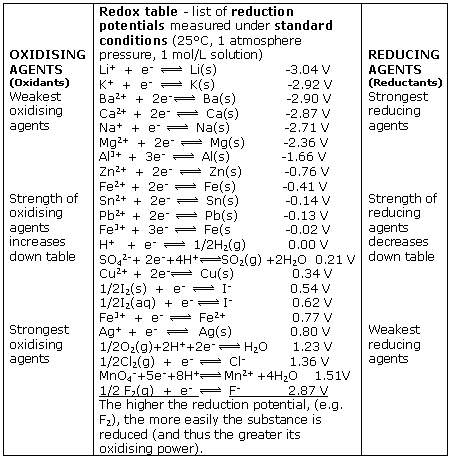
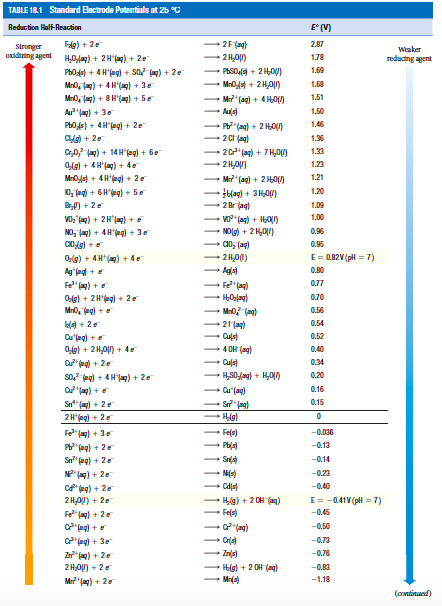
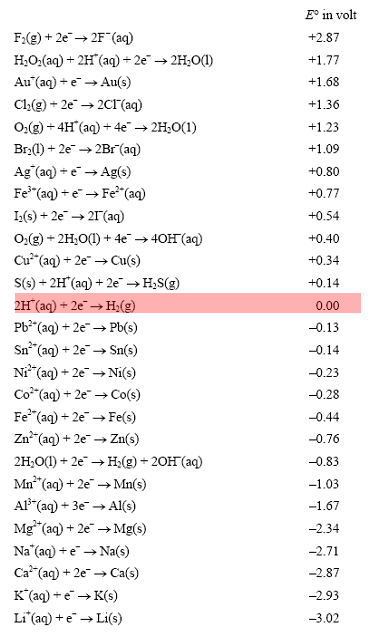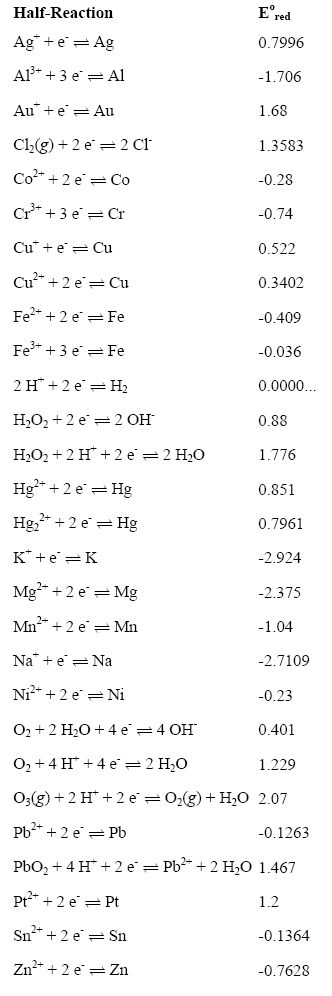Explain the difference between the electrochemical series and metal activity series Explain why sodium is placed below calcium in electrochemical - Chemistry - Electrolysis - 13786859 | Meritnation.com

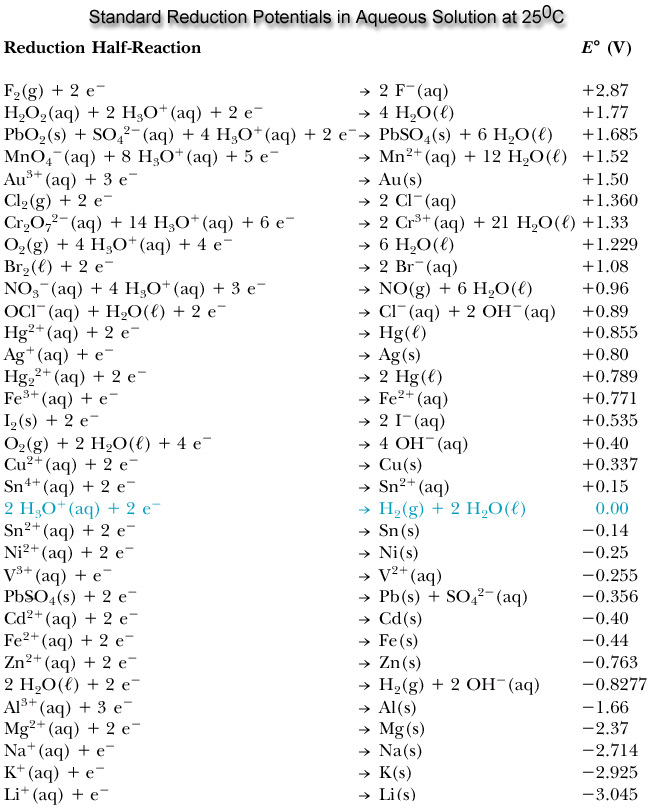
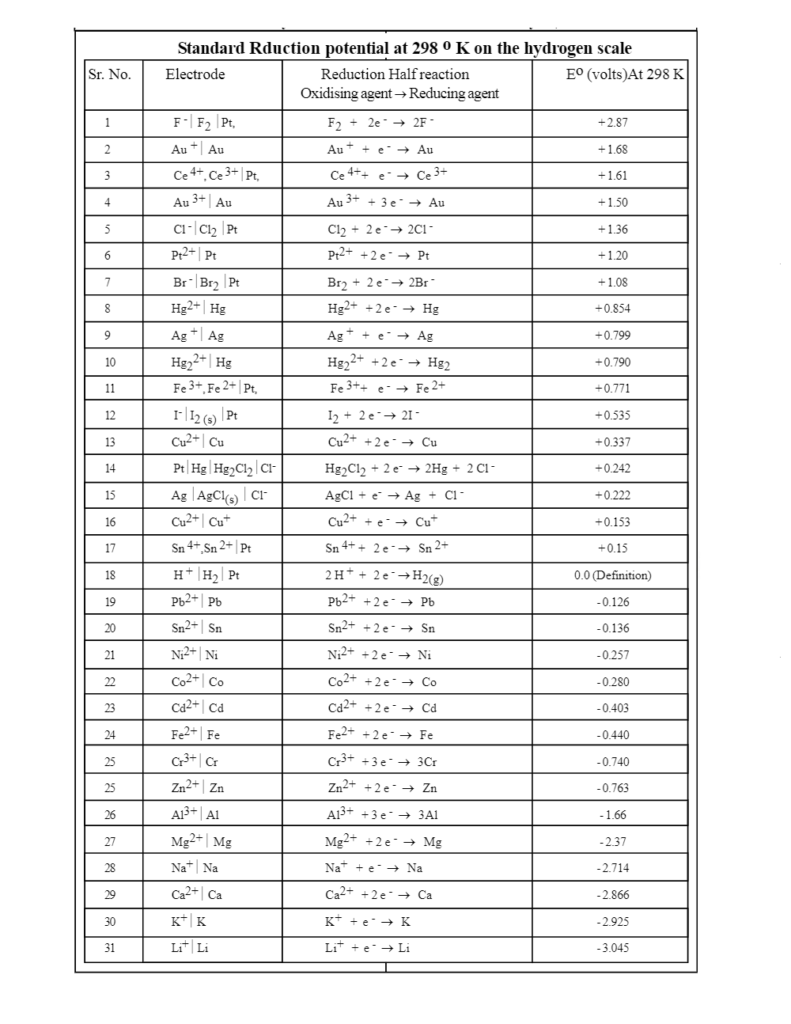
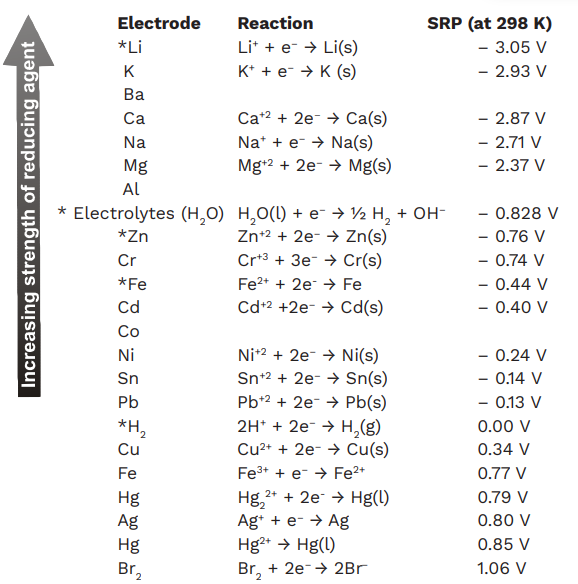
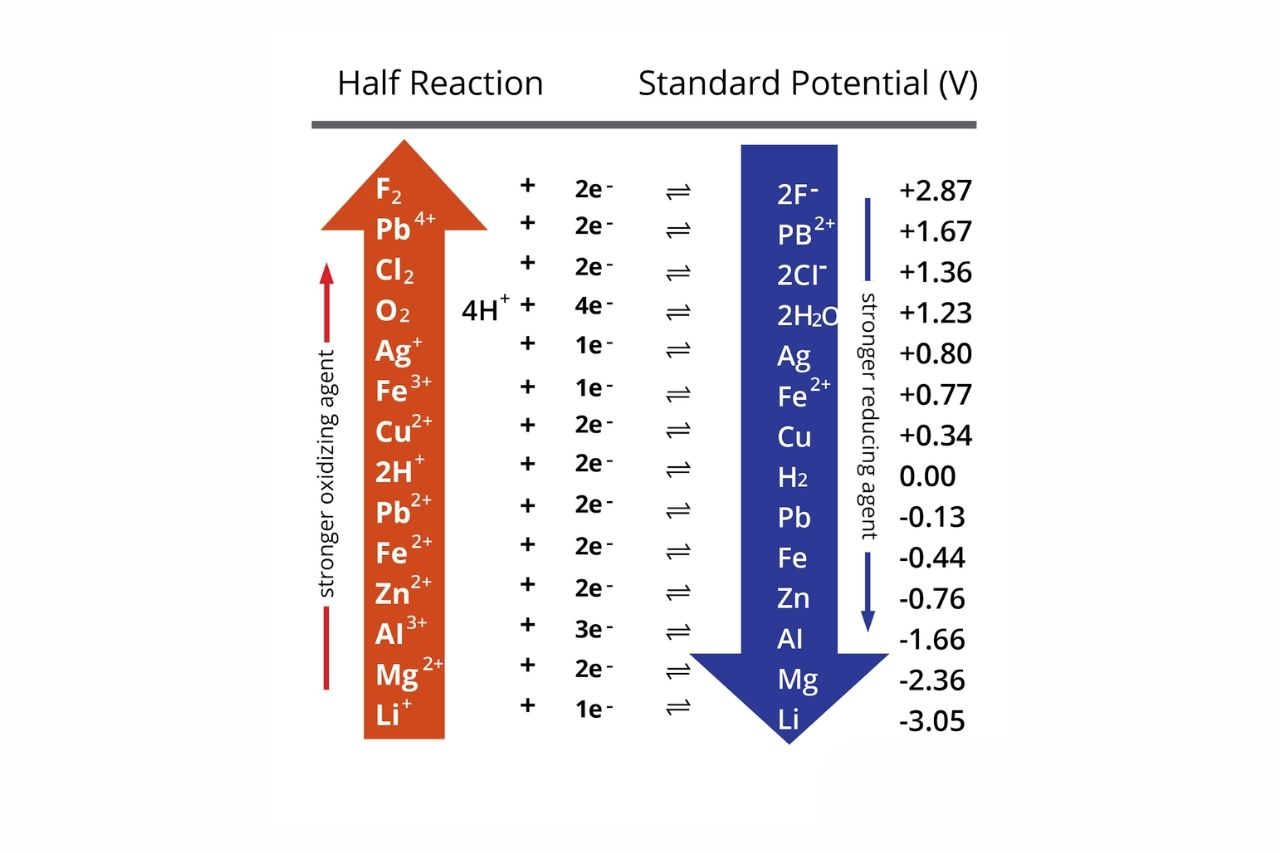
Basic Chemistry - “ELECTROCHEMICAL SERIES AND ITS APPLICATION:- A list of elements arranged in order on the basis of their standard reduction potential or oxidation potential is called electrochemical series. EXPLAINATION:- Different